Advertisements
IIT-JEE 2009 Chemistry Paper II Questions AndAnswers
PART�I:CHEMISTRY
PAPER -IISECTION �IStraight ObjectiveType
This section contains 4 multiple choicequestions. Each question has 4 choices(A), (B), (C) and (D), out of which ONE is correct.For the benefit of 11th/12th Studying students, we have (*)marked the questions which are from 11thsyllabus.You are advised to solve these questions in 100minutes.
1. For a first order reactionA → P, the temperature (T) dependent rate constant (k) wasfound to follow the equation log k = �(2000)1/T = 6.0.Thepre-exponential factor A and the activation energy Ea, respectively, are
(A) 1.0 �106s-1 and 9.2 kJmol�1 (B)6.0 s-1 and 16.6 kJmol�1
(C) 1.0 � 106s-1 and 16.6 kJmol�1 (D) 1.0 �106 s-1 and 38.3 kJmol�1
Key. (D)
Sol. K =Ae(-Ea/Rt) LogK= Log A -Ea/2.303RT
Log A = 6, A = 106s�1
E /2.303 *8.3*T = 2000/T
So Ea =38.3KJ
2. The spin only magnetic moment value(in Bohr magneton units) of Cr(CO)6 is
(A)0(B) 2.84
(C)4.90(D) 5.92
Key. (A)
Sol.
 Cr(CO)6
Cr(CO)6
Cr(zero)
Atomic configuration : 1s2 2s22p6 3s2 3p6 4s1 3d5
CO is a strong fieldligand
Configurationt2g
No. of unpaired electron =0
magnetic moment =0
*3. In the followingcarbocation, H/CH3 that is most likely tomigrate to the positively charged carbonis

(A)CH3 atC-4 (B) Hat C-4
(C) CH3 atC-2 (D) Hat C-2
Key. (D)
Sol. Hydride shift from C-2 willyield resonance stabilized 2�-carbocation giving thereby ketonic productafter deprotonation.
*4. The correct stabilityorder of the following resonance structuresis
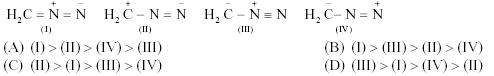
Key. (B)
Sol. In I and III all the atomsfulfill the octet requirement.Between II and IV, structure II hasnegative charge on nitrogen atom. Whereas in IV �ve chargeoccurs at
carbon which is lesselectronegative.
SECTION �IIMultiple Correct AnswerType
This section contains 5multiple correct answer's type questions. Each question has 4 choices (A), (B), (C) and (D), out of whichONE OR MORE is/are correct.
5. For the reduction ofNO3-ion in an aqueous solution E is +0.96 V.Values of E� for some metal ions are given below
V2+ (aq) + 2e� → V E0 =�1.19V
Fe3+ (aq) + 3e� → Fe E0 =�0.04V
Au3+ (aq) + 3e� → Au E0 = +1.40V
Hg2+ (aq) + 2e� → Hg E0 =+0.86V
The pair(s) of metals that is(are) oxidized byNO3� in aqueous solutionis(are)
(A) V and Hg (B) Hg and Fe
(C) Fe and Au (D) Fe andV
Key. (A, B,D)
Sol.NO3- ion will oxidise all those metal ions whosereduction E is less than 0.96V
*6. Among thefollowing, the state function(s) is(are)
(A) Internalenergy (B)Irreversible expansion work
(c) Reversible expansionwork (D)Molar enthalpy
Key. (A,D)
Sol. ∆E and ∆H are path independent andare definite quantities in a given change of states. Hence, E andH are State function
*7. In the reaction theamine(s) X is(are)

(A)NH3 (B)CH3NH2
(C)(CH3)2NH(D) (CH3)3N
Key. (A, B,C)
Sol. 3�-Amine form somedifferent kind of complex withdiborane
8. The nitrogen oxide(s) that contain(s) N-N bond(s)is(are)
(A)N2O(B)N2O3
(C)N2O4(D) N2O5
Key:(A,B,C)
Sol:
9. The correct statement(s) about the following sugars X and Yis(are)
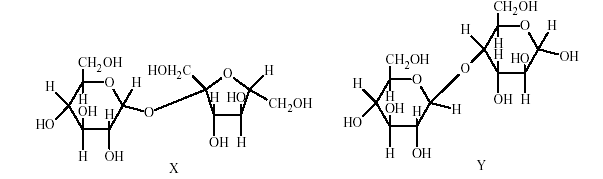
(A) X is a reducingsugar and Y is a non-reducing sugar
(B) X is a non-reducing sugarand Y is a reducing sugar
(C) The glucosidic linkages inX and Y are α and β,respectively
(D) The glucosidic linkages inX and Y are β and α,respectively
Key. (B,C)
Sol. In �X� the glycosidiclinkage is inbetween two anomeric C-atom while in Y it is only with oneanomeric
carbon, the other one is free. So, �X� will be non-reducingwhile �Y� will be reducing. Again the glycosidic
linkage in X is in betweenα-glucose and α-fructose, In Y, one of the glucose unit isα.
Hence (B) and (C)
SECTION� IIIMatrix MatchType
| Thissection contains 2 questions. Each question contains statements givenin twocolumns, which have to be matched. The statements in Column Iare labeled A, B, Cand D, while the statements in Column IIare labelled p, q, r, s and t. Any given statement in Column I can havecorrect matching with ONE OR MORE statement(s) in ColumnII. The appropriate bubbles correspondingto the answers to these questions have to be darkened as illustrated inthe following example: If the correctmatches are A � p, s and t; B � q and r; C � p and q; and D � s and t; thenthe correct darkening of bubbles will look like thefollowing: |  |
10. Match each of thereactions given in Column I with the corresponding product(s) given in ColumnII.
ColumnI ColumnII
(A) Cu + dilHNO3
| (p) NO |
(B)Cu + conc HNO3
| (q) NO2
|
(C) Zn + dilHNO3
| (r) N2O
|
(D)Zn + concHNO3
| (s) Cu(NO3)2 (t)Zn(NO3)2
|
Key. (A � p, s), (B �q, s), (C � r, t), (D � q, t)
Sol.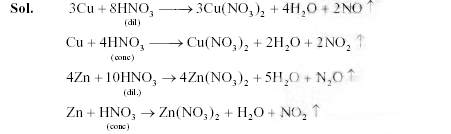
11. Match each of thecompounds given in Column I with the reaction(s), that they canundergo, given in
Column II.
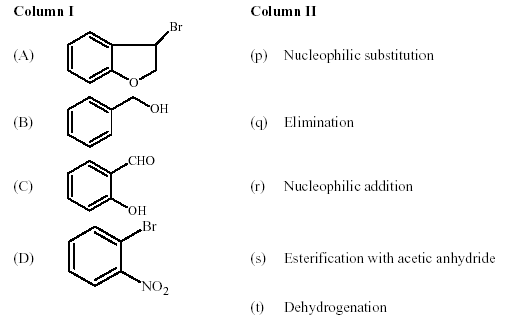
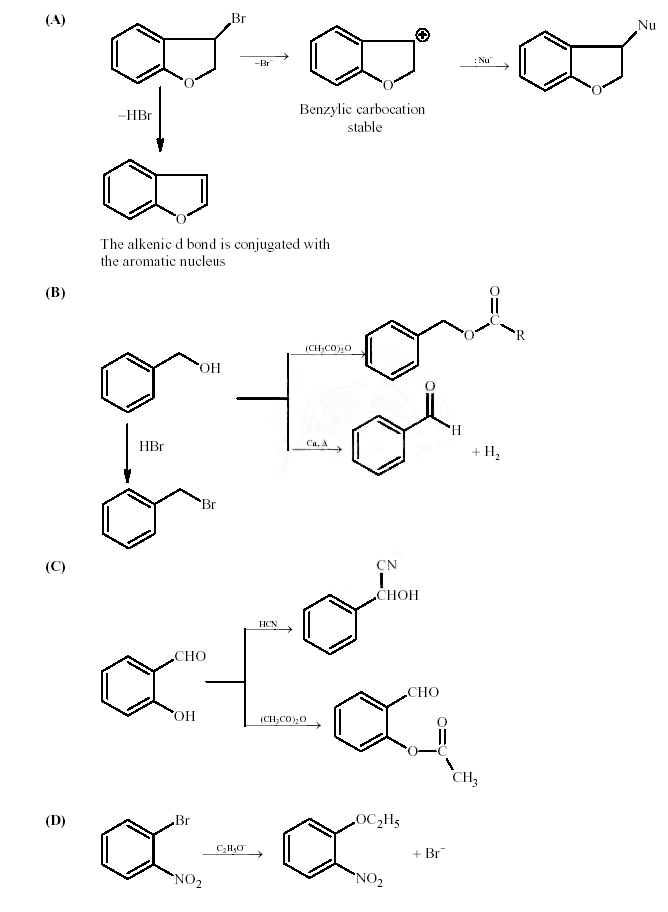
Key. (A � p,q), (B � p, s, t), (C � r, s), (D �p)
SECTION �IV
Integer AnswerType
Thissection contains 8 questions. The answer to each of the question is a singledigit integer, ranging from 0 to 9. The appropriate bubbles below the respective question numbers in the ORS have to be darkened.For example, if the correct answers to question numbers X, Y, Z and W (say) are6, 0, 9 and 2, respectively, then the correct darkening of bubbles will looklike the following:
*12. In a constant volumecalorimeter, 3.5 g of a gas with molecular weight 28 was burntin excess oxygen at
298.0 K. The temperature ofthe calorimeter was found to increase from 298.0 K to 298.45 K due tothe
combustion process. Given that the heat capacity of thecalorimeter is 2.5 kJ K�1, the numerical valuefor
the enthalpy of combustion of the gas in kJ mol�1is
Key. 9 kJ mol�1
Sol.Rise in temperature (293.45 � 298)= 0.45K
Heat evolved = 0.45 � 2.5 = 1.125kJ
No. of moles 3.5 1mol
= 28 8
Enthalpy ofcombustion
= 8 � 1.125
= 9kJ/moles
*13. At 400K, the root mean square (rms)speed of a gas X (molecular weight = 40) is equal to themost probable
speed of gas Y at 60 K. Themolecular weight of the gas Y is
Key. 4gmol�1
Sol. Ums = √3RT/M
Ump = √2RT/M
Fr4om EquationsM=4
*14. The dissociation constantof a substituted benzoic acid at 25�C is 1.0 � 10-4.The pH of a 0.01 M solution of its sodium saltis
Key. 8
Sol. pH = 7+ 0.5pKa+0.5 logC
=7+0.5*4+0.5log0.001
= 8
*15. The total numberof α and β particles emitted in the nuclearreaction 92U238 →82pb214
Key.8
SOl :No. of α particle = 238-214/4=6α
No. of β particle =2β
Total particle = 6 + 2 =8
16. The oxidationnumber of Mn in the product of alkaline oxidative fusion of MnO2 is
Key.6
Sol:
.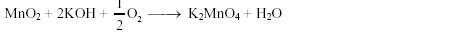
Oxidation state of Mn is +6
17. The number of watermolecule(s) directly bonded to the metal centre inCuSO4 . 5H2Ois
Key. 4
Sol. The structure ofCuSO4 . 5H2O isas follows
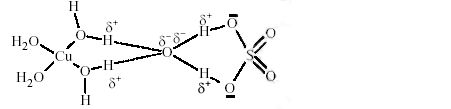
That is only four watermolecules are coordinated to central Cu2+ ion. One H2O moleculeexists H-bonded.
Hence answer is4.
*18. The coordination number of Al in thecrystalline state of AlCl3is
Key. 6
Sol. At lowtemperature AlCl3 exists a closed packed lattice of Cl� ions having Al3+ ionin octahedral void.
Hence C.N. issix.
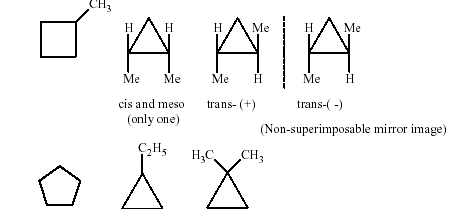
*19. The total number of cyclic structural as wellas stereo isomers possible for a compound with themolecular formulaC5H10is
Key.7
Sol. Total number of cyclic isomers ofC5H10 is7.